Russian Chemists Improve Seawater Desalination Membrane

A team of researchers of the HSE Faculty of Chemistry Joint Department of Inorganic Chemistry and Materials Science with the RAS Kurnakov Institute of General and Inorganic Chemistry have designed a novel type of hybrid ion-exchange membrane. Such membranes can be used to produce drinking water from seawater, which is particularly relevant for areas with access to the sea and a shortage of drinking water. The study is published in Desalination.
Membrane filters are used to filter drinking water and are considered the most advanced filtration systems today. However, modern membranes are not yet capable of entirely removing excess salts.
Seawater comprises various salts, including those which contain positively charged cations of sodium (Na+) and calcium (Ca2+) and negatively charged anions such as chlorides (Cl-) and sulphates (SO42-). Depending on the type of filtering membrane, either cations or anions can permeate it during desalination. In either case, membranes tend to be selective, eg by being more permeable to sodium than calcium.
Most anion exchange membranes are selective to doubly charged anions, ie sulphates (SO42-) or carbonates (CO32-). Such anions are more likely to cause a precipitation reaction. On an industrial scale, this can lead to failure of water treatment plants due to excessive saline deposits in their concentrate modules. Designing anion-exchange membranes which are selective to singly charged anions (chlorides Cl- and nitrates NO3-) is therefore an important R&D area in industrial water treatment.
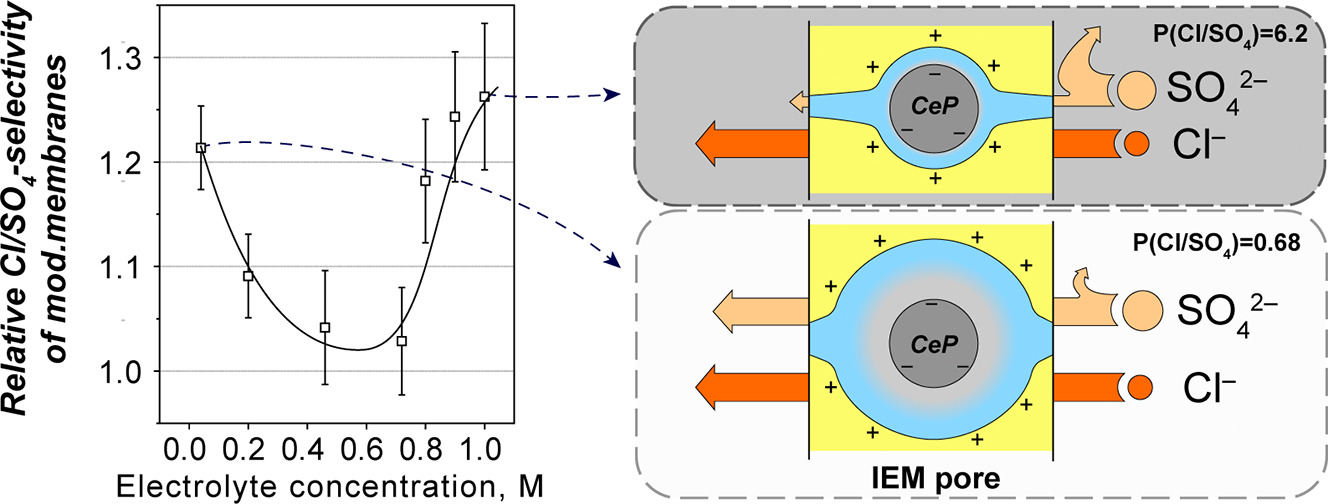
A team of researchers from the HSE Faculty of Chemistry and the RAS Institute of General and Inorganic Chemistry have come up with a novel approach to obtaining anion-exchange membranes which are selective to singly charged ions. The researchers modified ready-made membranes by introducing cerium phosphate containing acidic phosphate groups to the membrane pores to improve its properties. Since cerium phosphate dissolves in water poorly, it helps filter out particles and increase membranes' lifetime.
First, the membrane surface is treated with a cerium salt solution and then with a phosphoric acid salt. A chemical reaction between the salts produces cerium phosphate nanoparticles with negatively charged surfaces. Colliding with other negatively charged particles in seawater, they repel each other. According to Coulomb's law, the higher the negative charge, the stronger the repulsive force. Since sulphates have a -2 charge, they will be repelled more strongly from membrane pores containing cerium phosphate particles than, for example, singly charged chloride ions. This, according to the researchers, is one of the reasons behind the modified membranes' increased selectivity to singly charged anions.
The researchers varied the number and duration of the membrane treatment cycles and the concentration of reagents. The team also experimented with desalinating a mixed sodium chloride and sodium sulphate solution to determine which of the modified membranes was more selective to singly charged ions. The most impressive results—a 55% increase in the Cl/SO4-selectivity compared to the original commercial membrane—was shown by a modified membrane treated in one cycle with each of the reagents for 10 minutes.

Andrey Manin, co-author of the paper and student of the HSE Faculty of Chemistry
'We propose a fairly efficient and affordable method for obtaining anion-exchange membranes, and we hope that they will be used in the future for seawater desalination. We have recently been working as part of the Composite Materials for Environmental Protection research and study group to modify a different type of membrane using a similar method.'
See also:
Scientists Devise Cheaper and Easier Method for Synthesising Layered Rare Earth Hydroxides—'Chemical Sandwiches’
Researchers at HSE University and the RAS Kurnakov Institute of General and Inorganic Chemistry have developed a simplified and cost-effective method for synthesising layered rare earth hydroxides using propylene oxide. This reagent helps streamline the process and reduce its duration by several hours. In the future, this method is expected to facilitate the synthesis of various hydroxide-based hybrid materials, including photocatalysts for water purification and luminescent materials for solid-phase thermometers. The paper has been published in the Russian Journal of Inorganic Chemistry.
‘Digital Chemistry Is the Cutting Edge of Science’
In 2024, a new track ‘Digital Chemistry and Artificial Intelligence Technologies’ will open within the Bachelor’s programme in Chemistry. This track will offer courses in digital engineering, multi-scale modelling, chemometrics, and chemoinformatics, as well as big data and artificial intelligence technologies. Specialists from HSE University, Zelinsky Institute of Organic Chemistry, Moscow Institute of Physics and Technology, and Peter the Great St Petersburg Polytechnic University will be among the lecturers.
Chemists Improve Membranes for Water Treatment and Desalination
Chemists at HSE University, Kurnakov Institute of General and Inorganic Chemistry, and the University of Science and Technology of China have developed membranes with enhanced properties. The researchers experimentally revealed the impact of various factors on the desalination process and on the selectivity of ion separation. According to the study authors, their research will enable a more precise prediction of the properties of new ion-exchange membranes used in water treatment and desalination. The study findings have been published in Desalination.
‘The Main Thing Is to Try to Learn New Things by Any Honest Means’
Chemist Polina Yurova works in the same laboratory of the IGIC RAS that she first visited as a tenth grader. In this interview with the Young Scientists of HSE University project, she spoke about the creation of ion-exchange membranes, the ‘hair’ of black holes and her favourite Moscow park.
Russian Scientists Present New Application for Nanophotonic Sensor
A joint research team from HSE, Skoltech, MPGU and MISIS has developed a compact sensor for biochemical analysis, opening up a new frontier in the development of the ‘lab-on-a-chip’. Using bovine serum albumin film as an example, the researchers proved that the chip surface can be adapted for selective analysis of multicomponent solutions. Along with enabling accurate blood tests with only 3 to 5 microliters of blood, the chip will help doctors to detect specific disease markers. The study was published in Analytical Chemistry.
Scientists Create Uniquely Stable Trimeric Model of Coronavirus Spike Transmembrane Domain
A team of Russian scientists, including HSE MIEM researchers, have presented a 3D model of SARS-CoV-2 S-protein transmembrane (TM) domain. Previously, the TM domain had only been believed to anchor the S-protein in its viral membrane without being involved in rearrangement and fusion with the host cell. Yet according to recent studies, the TM domain appears to have a function in the transmission of genetic information, but its role is not yet fully understood. The researchers believe that the model they have created can contribute to a better understanding of viral mechanisms and potentially lead to the development of novel antiviral drugs. The study has been published in the International Journal of Molecular Sciences.
Fluoride Additive to Boost Production of Sedatives
Russian researchers from HSE University and the Russian Academy of Sciences Nesmeyanov Institute of Organoelement Compounds have come up with a new method of enhancing the chemical reaction involved in producing gamma-aminobutyric acid (GABA) analogues used in sedative drugs. Adding fluoride to the catalyst more than doubled the yield of the pure product and increased the total reaction yield by 2.5 times. This approach is expected to make the production of certain drug components more efficient and less costly. The study has been published in the Journal of Organic Chemistry.
HSE University Launches New Master’s Programme in Chemistry
In 2023, the first Master's programme 'Chemistry of Molecular Systems and Materials' at the HSE Faculty of Chemistry will enrol students. Half of the study time will be devoted to research projects in the field of modern fundamental and applied research on the topics studied at five institutes of the Russian Academy of Sciences. Dmitrii Roitershtein, Academic Supervisor of the programme and Associate Professor at the Joint Department of Organic Chemistry with the RAS Zelinsky Institute of Organic Chemistry, spoke about the programme to the HSE News Service.
‘We Want to Share Our Research with the Whole World’
The HSE University Faculty of Chemistry opened three years ago, and its first intake of undergraduate students is set to graduate in 2023. These students have already demonstrated impressive results in their research—half of second and third-year students have publications in journals indexed in WoS and Scopus, almost a third of which are in Q1 journals. Andrey Yaroslavtsev, Head of the Chemistry programme and Academic of the Russian Academy of Sciences (RAS), told the HSE University News Service about the secret of this success.
New Data Gained on Double Perovskite Oxides
The Journal of Alloys and Compounds has published an article coauthored by the Institute of Solid State Chemistry and Mechanochemistry (the Ural Branch of the Russian Academy of Sciences), the Donostia International Physics Centre, and the HSE Tikhonov Moscow Institute of Electronics and Mathematics on the characteristics of cubic double perovskite oxides. To date, experimental measurements of the minerals’ characteristics have not corresponded to the results of theoretical modelling. The work marks the first time that researchers have set themselves the task of explaining this disparity. The data obtained will allow researchers to improve low-temperature fuel cell technologies—one of the main alternatives to current sources of electricity.


